New York, Sep 23 (IANS) In a ray of hope for people with alopecia areata — an autoimmune disease that causes patchy, and sometimes total hair loss – researchers have found promise in a drug which is already approved for treating certain bone marrow disorders.
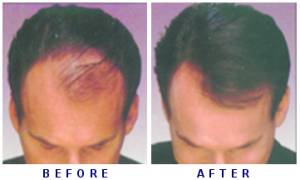
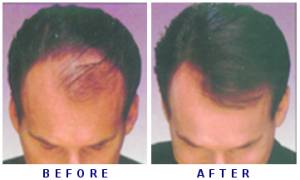
In a study, researchers from Columbia University Medical Center (CUMC) reported that seventy five percent of patients with moderate to severe alopecia areata had significant hair regrowth after treatment with ruxolitinib.
By the end of their treatment, average hair regrowth was 92 percent, said the study published in the Journal of Clinical Investigation/Insight.
Ruxolitinib is a US Food and Drug Administration (FDA)-approved drug that inhibits the Janus kinase (JAK) family of enzymes known as JAK inhibitors.
“Although our study was small, it provides crucial evidence that JAK inhibitors may constitute the first effective treatment for people with alopecia areata,” said Julian Mackay-Wiggan, Associate Professor at CUMC .
“This is encouraging news for patients who are coping with the physical and emotional effects of this disfiguring autoimmune disease,” Mackay-Wiggan noted.
Alopecia areata, the second most common form of hair loss, can occur at any age and affects men and women equally.
The disease usually causes hair loss on the scalp, but some patients also experience facial and body hair loss, with devastating consequences particularly in children. Currently, there is no known treatment that can completely restore hair.
Previously, the Columbia researchers identified the specific immune cells and the dominant inflammatory signalling pathways responsible for attacking the hair follicle in alopecia areata, putting them into a dormant state.
Subsequent experiments with mouse and human hair follicles showed that topical and oral drugs that inhibit the Janus kinase family of enzymes, reawaken these dormant follicles by blocking inflammatory signalling.
Two such JAK inhibitors already approved by the U.S. FDA are ruxolitinib, a medication that is used to treat bone marrow malignancies, and tofacitinib, a treatment for rheumatoid arthritis.
To test this hypothesis, the researchers initiated a small, open-label clinical trial of 12 patients with moderate to severe alopecia areata (more than 30 per cent hair loss).
All patients were given 20 mg of oral ruxolitinib, twice a day, for three to six months.
Participants were followed for an additional three months to assess the durability of treatment response.
Nine of the patients had hair regrowth of 50 per cent or more. By the end of the treatment period, 77 per cent of those who responded to the therapy achieved hair regrowth of over 95 per cent.
A third of the responders had significant hair loss in the follow-up period after the medication was stopped, although their hair loss did not reach pre-treatment levels.
The drug was well tolerated in all participants with no serious adverse effects, the researchers reported.